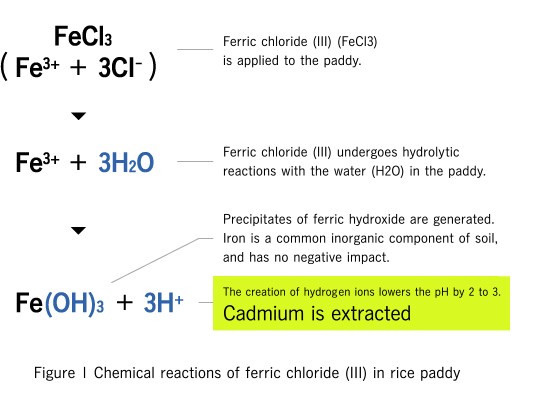
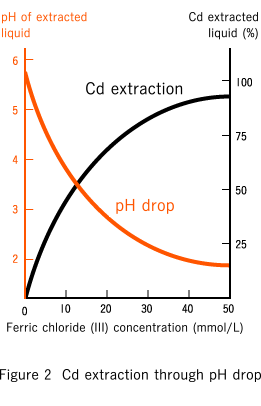
Mechanism of extracting cadmium using a cleaning agent (ferric chloride (III))
As the result of testing a large number of chemicals, we found that ferric chloride (III) was highly efficient at extracting cadmium from soil, at low cost, and with little negative impact on the soil. Ferric chloride (III) is water-soluble and produces ferric and chlorine ions. Ferric ions produce hydrogen ions as the result of hydrolytic reactions, allowing the cleaning agent to remove cadmium while maintaining optimum pH levels and remaining friendly to the environment. Chlorine ions form complex molecules with cadmium, promoting elution into the water.
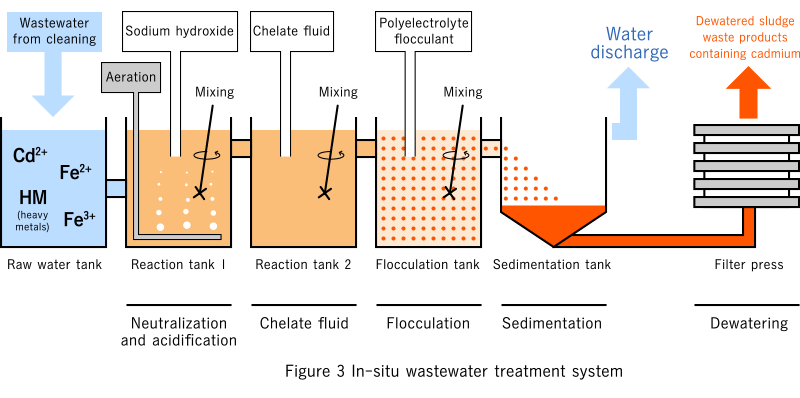
Wastewater treatment
Wastewater containing cadmium is treated by portable wastewater treatment equipment which contains a compact alkali coagulation sedimentation system.
Heavy metals in wastewater such as cadmium are collected as sediment and processed as dewatered sludge waste products.