Durability Prediction System
| >Introduction | >Overview | >Thermodynamic phase equilibrium model Mass transfer model |
>Features | >Example of durability prediction | >Future prospects |
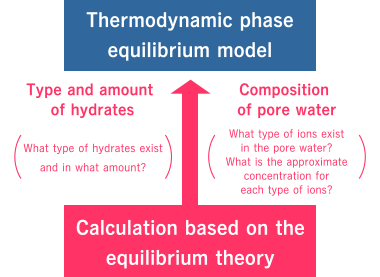
Thermodynamic phase equilibrium model
This model uses equilibrium theory as a basis to calculate the chemical species and solid phases in which substances of a certain system exist under given conditions (temperature, PH, redox state, etc.), as well as the approximate amount of those forms.
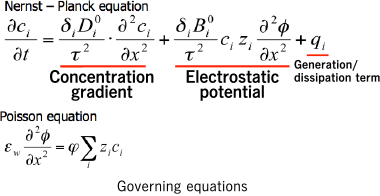
Mass transfer model
n this simulation, the movement of ions in the liquid phase is determined through numeric calculation using the Nernst-Planck equation and the Poisson equation as the governing equations. The Nernst-Planck equation applies to the conservation of mass, while the Poisson equation applies to the electrostatic potential which occurs in conjunction with transfer.
When performing coupled analysis using the Nernst-Planck equation and the Poisson equation, it is possible to use the finite element method for solutions. Here, the Galerkin method is used for discretization related to space, and the Θ method (Θ = 0.878) is used for discretization related to time.
Results of the thermodynamic equilibrium model are substituted for the generation/dissipation term of the Nernst-Planck equation. This couples the mass transfer model and the thermodynamic phase equilibrium model, thus making it possible to predict temporal changes in gas-phase, liquid-phase, and solid-phase composition.
When performing coupled analysis using the Nernst-Planck equation and the Poisson equation, it is possible to use the finite element method for solutions. Here, the Galerkin method is used for discretization related to space, and the Θ method (Θ = 0.878) is used for discretization related to time.
Results of the thermodynamic equilibrium model are substituted for the generation/dissipation term of the Nernst-Planck equation. This couples the mass transfer model and the thermodynamic phase equilibrium model, thus making it possible to predict temporal changes in gas-phase, liquid-phase, and solid-phase composition.